Details of the Drug
General Information of Drug (ID: DM0QV1J)
| Drug Name |
Baclofen
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arbaclofen; (R)-Baclofen; 69308-37-8; (R)-4-Amino-3-(4-chlorophenyl)butanoic acid; d-Baclofen; (-)-Baclofen; (R)-(-)-Baclofen; R-Baclofen; R-(-)-Baclofen; STX209; (3R)-4-amino-3-(4-chlorophenyl)butanoic acid; UNII-NYU6UTW25B; l-Baclofen; STX 209; (R)-4-Amino-3-(4-chlorophenyl)butyric Acid; NYU6UTW25B; CHEMBL301742; AK109161; Benzenepropanoic acid, beta-(aminomethyl)-4-chloro-, (R)-; Benzeneporopanoic acid, (beta-(aminomethyl)-4-chloro-, (betaR)-; Arbaclofen [USAN:INN]; R-Baclofen RS; Arbaclofen (USAN); p-chlorophenyl GABA; ApoBaclofen; Atrofen; Baclofene; BaclofeneIrex; Baclofeno; Baclofenum; Baclon; Baclophen; Baclospas; Clofen; Gabalon; GenBaclofen; Genpharm; Kemstro; Lebic; Lioresal; NuBaclo; PMSBaclofen; ASTA Medica Brand of Baclofen; Alphapharm Brand of Baclofen; Apo Baclofen; Apotex Brand of Baclofen; Ashbourne Brand of Baclofen; Athena Brand of Baclofen; Baclofen AWD; Baclofen Alphapharm Brand; Baclofen Apotex Brand; Baclofen Ashbourne Brand; Baclofen Athena Brand; Baclofen Irex Brand; Baclofen Isis Brand; Baclofen Medtronic Brand; Baclofen Novartis Brand; Baclofen Pharmascience Brand; Baclofene Irex; Chlorophenyl GABA; Ciba Geigy Brand of Baclofen; Gen Baclofen; Irex Brand of Baclofen; Isis Brand of Baclofen; Lioresal Intrathecal; Medtronic Brand of Baclofen; Novartis Brand of Baclofen; Nu Baclo; Nu Pharm Brand of Baclofen; PMS Baclofen; Pharmascience Brand of Baclofen; B 5399; Ba 34647; Ba34647; C 34647Ba; AWD, Baclofen; Apo-Baclofen; Ba-34647; Ba34,647; Baclofen Ciba-Geigy Brand; Baclofen Nu-Pharm Brand; Baclofene [INN-French]; Baclofene-Irex; Baclofeno [INN-Spanish]; Baclofenum [INN-Latin]; CIBA34,647BA; Ciba-Geigy Brand of Baclofen; DL-Baclofen; GABA, Chlorophenyl; Gen-Baclofen; Kemstro (TN); Lioresal (TN); Nu-Baclo; Nu-Baclofen; Nu-Pharm Brand of Baclofen; PCP-GABA; Pms-Baclofen; Ba-34,647; Baclofen (R,S); Ciba 34,647-Ba; Baclofen (JP15/USP/INN); Baclofen [USAN:INN:BAN:JAN]; Beta-(4-Chlorophenyl)gaba; CIBA-34,647-BA; Beta-(Aminomethyl)-4-chlorobenzenepropanoic acid; Beta-(Aminomethyl)-p-chlorohydrocinnamic acid; Beta-(p-Chlorophenyl)-gamma-aminobutyric acid; DL-4-Amino-3-p-chlorophenylbutanoic acid; Gamma-Amino-beta-(p-chlorophenyl)butyric acid; Benzenepropanoic acid, beta-(aminomethyl)-4-chloro-(9CI); (+-)-Baclofen; (+/-)-BACLOFEN; (+/-)-beta-(Aminoethyl)-4-chlorobenzenepropanoic acid; (+/-)-beta-(Aminomethyl)-4-chlorobenzenepropanoic acid; (inverted question mark)-Baclofen; 4-Amino-3-(4-chlorophenyl)butanoic acid; 4-Amino-3-(4-chlorophenyl)butyric acid; (R)-4-Amino-3-(4-chloro-phenyl)-butyric acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Muscle Relaxants
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
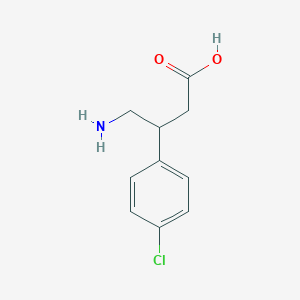 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 213.66 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Baclofen (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1084). | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01706523) Open Label Extension Study of STX209 (Arbaclofen) in Autism Spectrum Disorders. U.S. National Institutes of Health. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Synthesis and pharmacology of the baclofen homologues 5-amino-4-(4-chlorophenyl)pentanoic acid and the R- and S-enantiomers of 5-amino-3-(4-chlorop... J Med Chem. 1999 Jun 3;42(11):2053-9. | ||||
| 9 | Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007 Dec;24(12):2281-96. | ||||
| 10 | Anti-inflammatory effects of the GABA(B) receptor agonist baclofen in allergic contact dermatitis. Exp Dermatol. 2010 Jul 1;19(7):661-6. doi: 10.1111/j.1600-0625.2010.01076.x. Epub 2010 Feb 25. | ||||
| 11 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 12 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 13 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 14 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 15 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 16 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 17 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 18 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 19 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 20 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 21 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
| 22 | Product Information. Zyrtec (cetirizine). Pfizer US Pharmaceuticals, New York, NY. | ||||
